Preparation and Patient Administration of XENOVIEW®
- XENOVIEW® is prepared in the HPX Hyperpolarizer according to the HPX Hyperpolarization System Operater’s Manual and dispensed into the XENOVIEW® Dose Delivery Bag
- The recommended dose of XENOVIEW® for adults and pediatric patients aged 12 years and older is 75 mL to 100 mL dose equivalent (DE) of hyperpolarized Xenon Xe 129 Gas Blend by oral inhalation of the entire contents of one XENOVIEW® Dose Delivery Bag
- The volume and DE in the XENOVIEW® Dose Delivery Bag are measured using the HPX Polarization Measurement Station no more than 5 minutes prior to patient administration
- The dose is administered within 5 minutes of DE measurement (discard the dose of XENOVIEW® 60 minutes after hyperpolarization)
Administration of XENOVIEW® and image acquisition is a 3-step process that takes place in the magnetic resonance imaging (MRI) suite:

Not an actual patient.
Patient is fitted with a XENOVIEW® 3.0T Chest Coil and positioned supine inside the MRI scanner.
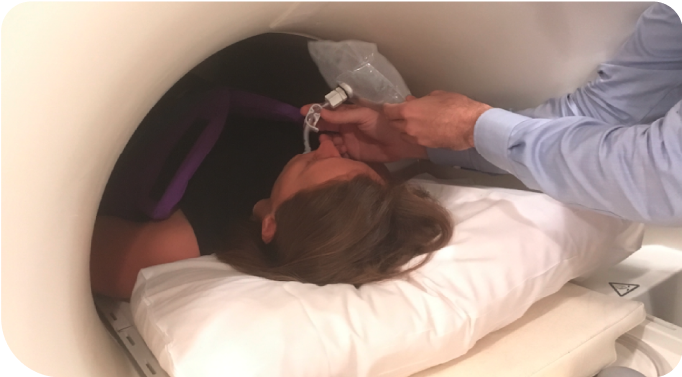
Not an actual patient or technician.
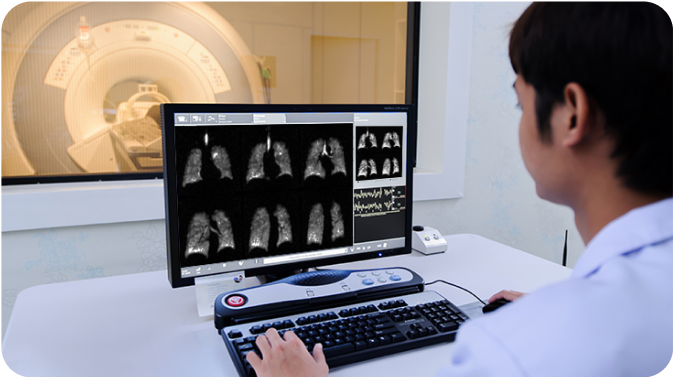
Not an actual patient or technician.
MRI scanning begins immediately and is completed in a single 10 to 15 second breath hold.
